Scientific Calendar August 2023
Accurate monitoring of haemophilia treatment
Which APTT reagent should not be used to monitor therapy with Esperoct?
The choice of the APTT reagent is not limited in any way. Any reagent, whether based on silica, ellagic acid or kaolin, can be used to monitor the therapy with Esperoct.
Most silica-based APTT reagents may lead to an underestimation of the concentration of infused Esperoct. The choice of APTT reagent therefore depends on the specific product.
Congratulations!
That's the correct answer!
Sorry! That´s not completely correct!
Please try again
Sorry! That's not the correct answer!
Please try again
Notice
Please select at least one answer
Background
1 Not recommended by some guidelines
2 Product-specific calibrator recommended
3 Discrepant results between studies; guideline recommends using chromogenic based assays and results must be interpreted with caution or compared with chromogenic assay results if one-stage APTT-based factor assay is determined
4 Using a modified assay protocol with higher sample dilution and specific Emicizumab calibrator
? Not specifically supported or excluded
While haemophilia as such has been known for many centuries and described by various scholars, the history of successful treatment is still relatively young. The first treatment options included only the acute treatment of present bleeding through the administration of whole blood donations from relatives and, with the discovery of clotting factors, the administration of plasma transfusions. However, this type of treatment could not yet prevent bleeding. This only changed from the 1960s onwards, when Judith G. Pool discovered that plasma cryoprecipitates contain large amounts of factor VIII (FVIII). Even though these cryoprecipitates continued to be used to treat existing bleeding in the early years, the discovery laid the foundation for preventive therapy, which would be achieved for the first time with the development of recombinant factor concentrates in the second half of the 1980s. [1, 2]
In recent years, the development of preparations for the therapy of haemophilia has gained new momentum. New recombinant products with an extended half-life (EHL), based on modification of the respective coagulation factor, modification of the coagulation factor by fusion of the Fc region of IgG1 immunoglobulin (-Fc) or albumin, or the addition of polyethylene glycol (PEG) with different molecular weights, have become available as options in therapy. [3] However, therapy strategies not aimed at factor replacement therapy are also becoming increasingly widespread. Emicizumab, for example, is a humanised monoclonal bispecific antibody for the treatment of haemophilia A (HA). One side of the antibody binds to activated coagulation factor IX (FIXa) and the other to coagulation factor X (FX). It thus mimics the function of activated factor VIII (FVIIIa), which is absent or reduced in HA patients. [4] Another option for the therapy of haemophilia patients is gene therapy. Here, a fully functional copy of the coagulation factor gene of the gene defective in haemophilia is introduced into the patient's body by means of genetically modified viruses (usually adeno-associated viruses (AAV)). Translation of the gene into endothelial cells and sinusoidal cells of the liver is stimulated by a promoter so that the patient can ideally produce sufficient clotting factor concentrations. [5]
Reliably and accurately monitoring the treatment of people with haemophilia is of great importance to avoid unwanted bleeding and long-term sequelae.
One- and two-step coagulation assays as well as chromogenic assays (CSA) are available for the determination of FVIII and FIX levels. Differences in the results of the various assays in hereditary haemophilia, especially in mild forms, are well known. [6, 7, 8, 9, 10] The same applies to the results for therapy monitoring with human-derived factor concentrates and full-length recombinant products, even though single-step coagulation tests and chromogenic tests essentially allow accurate therapy monitoring. With the development of new EHL recombinant coagulation factors and the so-called non-factor replacement therapeutics, new challenges for the laboratory arise as the laboratory should use an assay for therapy monitoring where the results are close to the calculated treatment value based on the drug label.
Differences in the results of different one-step APTT-based factor (OSA) tests are attributed to various factors. The choice of APTT reagent to determine factor activity is hereby a factor. APTT reagents differ in terms of the phospholipids used (of plant or animal origin or recombinant) and their concentration as well as the choice of activator. In addition, the choice of factor deficiency plasma, eg the von Willebrand factor (VWF) concentration of FVIII deficiency plasmas or factor residual activity of the deficiency plasma, also has a critical influence on determining factor concentration in haemophilia patients. Other factors that can influence the quality of haemophilia therapy monitoring are the choice of calibrator, with assay specific calibrators being recommended in some cases, the analyser, and the test protocols (single-dilution analysis or multi-dilution analysis and linearity of the test). [10, 11, 12]
As mentioned above, discrepancies exist in the determination of factor activity between the OSA’s and CSA’s when determined by these two methods in patients with mild haemophilia. [6, 7, 8, 9, 10] In general, the results obtained with OSA’s are higher than the CSA’s, especially in patients with mild HA. However, the opposite can also be true, in which the chromogenic result corresponds to mild haemophilia, but the clinical picture contradicts this. [13]
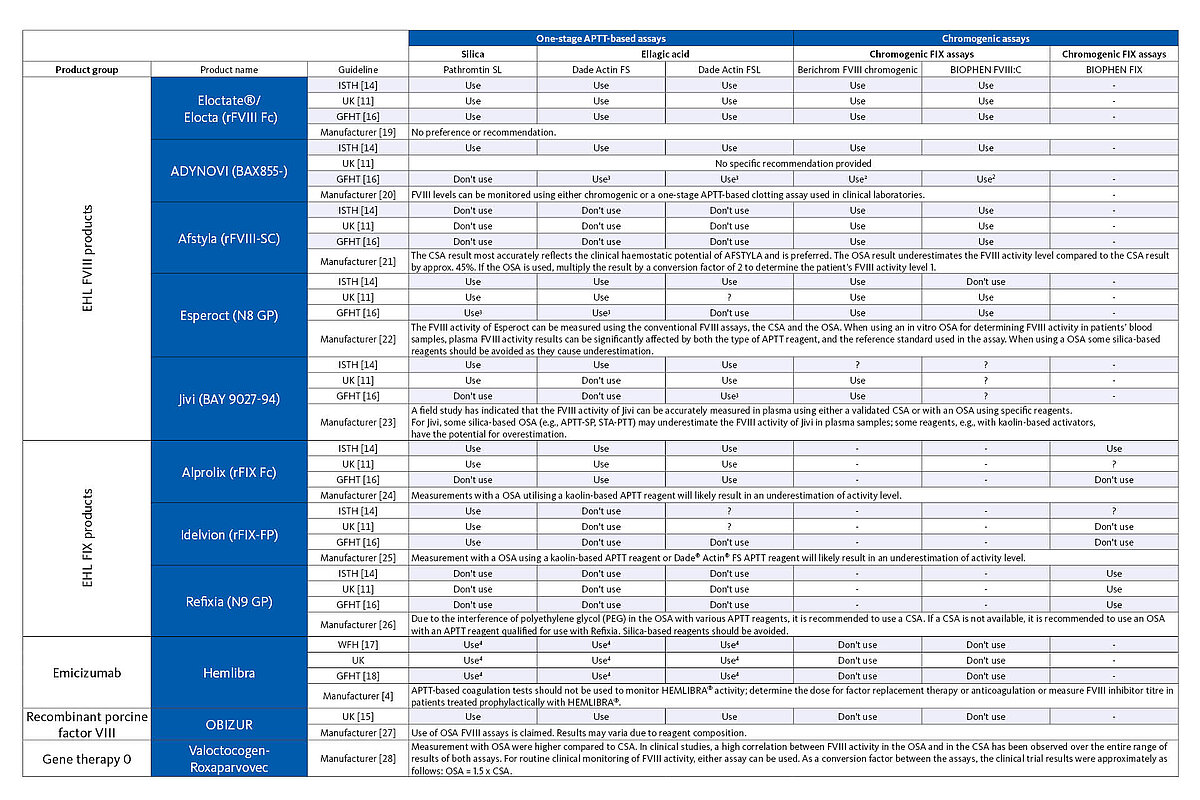
Another factor in monitoring haemophilia therapy that should not be neglected is the drug itself. For some drugs with EHL, OSA’s are not recommended because they underestimate factor activity. For some other drugs, this limitation only applies to the activator used by the APTT reagent. For example, when determining the infused FVIII concentration of Esperoct (N8 GP), most silica-based APTT reagents should not be used because the FVIII concentration is underestimated when using this reagent, which is not the case with other, non-silica-based APTT reagents. However, it must be noted that individual guidelines also exclude some non-silica-based APTT reagents from therapy monitoring of Esperoct. For other recombinant products, the choice of assay method is not important as they can be measured accurately with both OSA and CSA.
Given the large number of different therapy approaches, it is important for the laboratory to know which drug the haemophilia patient has received in order to choose the most suitable measurement method for therapy monitoring. The table below gives an overview of the test systems that should be used for therapy monitoring of the different haemophilia therapy options according to the manufacturer's instructions and expert opinion.
References
[1] Schramm W. (2014): The history of haemophilia - a short review. Thromb Res. 2014 Nov;134 Suppl 1: S4-9.
[2] Franchini M, Mannucci PM. (2014): The history of hemophilia. Semin Thromb Hemost. 2014 Jul;40(5):571-6.
[3] Young G, Mahlangu JN. (2016): Extended half-life clotting factor concentrates: results from published clinical trials. 2016 Jul;22 Suppl 5:25-30.
[4] Hemlibra® (2022): Leitfaden für medizinisches Fachpersonal, Version 3.0, Stand: Dezember 2022. Roche Pharma AG.
[5] ROCTAVIAN (2023): SUMMARY OF PRODUCT CHARACTERISTICS. Retrieved from EMA, 26 June 2023.
[6] Bowyer AE, Van Veen JJ, Goodeve AC, Kitchen S, MakrisM. (2013): Specific and global coagulation assays in the diagnosis of discrepant mild hemophilia A. Haematologica 2013;98(12):1980–1987
[7] Baig MA, Swamy KB. (2021): Comparative analysis of chromogenic vs clot-based one stage APTT assay for determination of factor VIII level. Indian J Pathol Microbiol. 2021 Jan-Mar;64(1):123-127.
[8] Potgieter, J.J., Damgaard, M. and Hillarp, A. (2015): One-stage vs. chromogenic assays in haemophilia A. Eur J Haematol, 94: 38-44.
[9] Annette E. Bowyer, Robert C. Gosselin. (2022): Factor VIII and Factor IX Activity Measurements for Hemophilia Diagnosis and Related Treatments. Semin Thromb Hemost, available online at DOI: 10.1055/s-0042-1758870.
[10] Pouplard C, Trossaert M, Le Querrec A, et al. (2009): Influence of source of phospholipids for APTT- based factor IX assays and potential consequences for the diagnosis of mild haemophilia B. Haemophilia. 2009;15(1):365-368.
[11] Gray E, Kitchen S, Bowyer A, et al. (2020): Laboratory measurement of factor replacement therapies in the treatment of congenital haemophilia: A United Kingdom Haemophilia Centre Doctors’ Organisation guideline. Haemophilia. 2020; 26:6–16.
[12] Nougier C, Sobas F, Nguyen TK, et al. (2011): Analytic variability due to change of deficient plasma vials: application to one-stage clotting factor VIII assay. Blood Coagul Fibrinolysis 2011;22: 151–154.
[13] Bowyer AE, Goodeve A, Liesner R,Mumford AD, Kitchen S, Makris M. (2011): p.Tyr365Cys change in factor VIII: haemophilia A, but not as we know it. Br J Haematol 2011;154(5):618–625.
[14] Peyvandi F, et al. (2020): Laboratory testing in hemophilia: Impact of factor and non-factor replacement therapy on coagulation assays. J Thromb Haemost.; 18: 1242–1255.
[15] Bowyer A, Gray E, Lowe A, et al. (2022): Laboratory coagulation tests and recombinant porcine factor VIII: A United Kingdom Haemophilia Centre Doctors’ Organisation guideline. Haemophilia. 2022;1-5.
[16] Jeanpierre E, Pouplard C, Lasne D, et al; On behalf of the French Study Group on the Biology of Hemorrhagic Diseases (the BIMHO group). (2020): Factor VIII and IX assays for post-infusion monitoring in hemophilia patients: Guidelines from the French BIMHO group (GFHT). Eur J Haematol. 2020; 105:103–115.
[17] Srivastava A, et al. (2020): WFH Guidelines for the Management of Hemophilia, 3rd edition. Haemophilia: 26 (Suppl 6): 1 – 158.
[18] Nougier C, et al. (2020): Emicizumab treatment: Impact on coagulation tests and biological monitoring of haemostasis according to clinical situations (BIMHO group proposals). Eur J Haematol.; 105: 675–681.
[19] Elocta (2023): SUMMARY OF PRODUCT CHARACTERISTICS. Retrieved from EMA, 26 June 2023.
[20] ADYNOVI (2023): SUMMARY OF PRODUCT CHARACTERISTICS. Retrieved from EMA, 26 June 2023.
[21] AFSTYLA (2023): SUMMARY OF PRODUCT CHARACTERISTICS. Retrieved from EMA, 26 June 2023.
[22] Esperoct (2023): SUMMARY OF PRODUCT CHARACTERISTICS. Retrieved from EMA, 26 June 2023.
[23] Jivi (2023): SUMMARY OF PRODUCT CHARACTERISTICS. Retrieved from EMA, 26 June 2023.
[24] Alprolix (2023): SUMMARY OF PRODUCT CHARACTERISTICS. Retrieved from EMA, 26 June 2023.
[25] Idelvion (2023): SUMMARY OF PRODUCT CHARACTERISTICS. Retrieved from EMA, 26 June 2023.
[26] Refixia (2023): SUMMARY OF PRODUCT CHARACTERISTICS. Retrieved from EMA, 26 June 2023.
[27] OBIZUR (2023): SUMMARY OF PRODUCT CHARACTERISTICS. Retrieved from EMA, 26 June 2023.
[28] Valoctocogen-Roxaparvovec (2023): SUMMARY OF PRODUCT CHARACTERISTICS. Retrieved from EMA, 26 June 2023.