Scientific Calendar July 2023
Haemolytic uraemic syndrome caused by Shiga toxin-producing E. coli
Where in the RET channel scattergram are fragmented RBC located?
in the light blue area at the bottom of the scattergram
in the enlarged red area with high side fluorescence signals (SFL)
in the extended dark blue RBC area towards the low forward scattered light (FSC)
fragmented RBC can be only seen in the RBC histogram
Congratulations!
That's the correct answer!
Sorry! That´s not completely correct!
Please try again
Sorry! That's not the correct answer!
Please try again
Notice
Please select at least one answer
Case study and results interpretation
A two-year-old patient presented at the hospital with watery stool, which later became bloody with fever appearing.
The blood count showed an HGB of 6.8 g/dL (4.22 mmol/L), resulting in an ‘Anaemia’ flag on the analyser and a PLT-F count of 27 x109/L triggering the ‘Thrombocytopenia’ flag. On day 4 after admission, schistocytes were present at 4.0% on the blood smear.
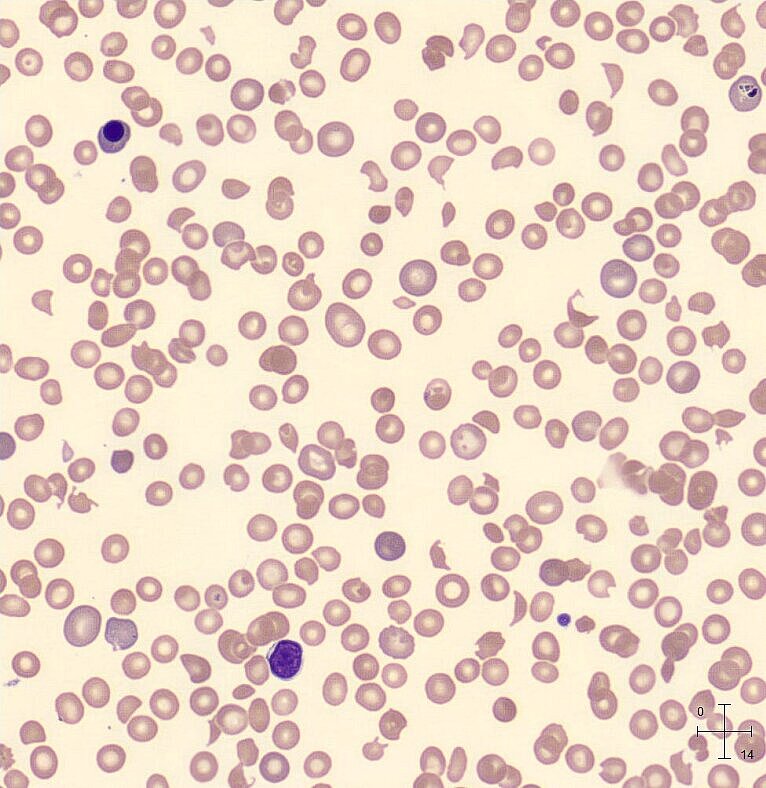
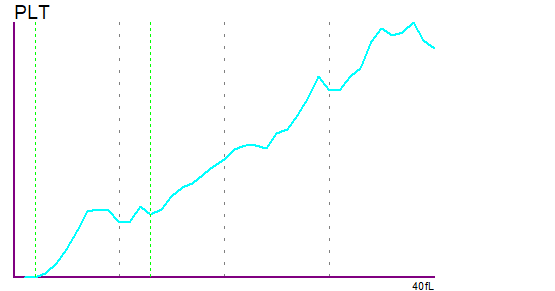
Interferences of the schistocytes were seen in the PLT histogram, which triggered the ‘PLT Abn Distribution’ flag.
The reticulocyte population seen in the RET scattergram was increased (purple and red area). Additionally, there are blue particles extending from the mature RBC cluster to the low FSC area. Presence of small-sized red blood cells and schistocytes in this area of the scattergram triggered the flag ‘Fragments?’.
RET scattergram with an RBC population (blue) extended towards the low FSC area.
Testing results further showed acute kidney injury and elevated lactate dehydrogenase (LD), as well as an increase in WBC and C-reactive protein (CRP). This led to the suspicion of HUS. Antibodies to Escherichia coli O157 lipopolysaccharide were found, confirming the diagnosis of with HUS due to Shiga toxin-producing E. coli.
Scientific Background
Strategy one: The colonisation
Escherichia coli is a pathogen of the human gastrointestinal tract which has been known to cause food-borne infections, with outbreaks first reported in 1983 [1, 2].
E. coli O157 is the most predominant serotype, causing approximately one million cases of diarrhoea and 2,000 cases of haemolytic uraemic syndrome (HUS) worldwide per year [3]. It is a type of enterohaemorrhagic E. coli (EHEC) [4], which are known to have a chromosomal pathogenicity island encoding for a type 3 secretion system (T3SS), an adhesin named ‘intimin’ and its receptor ‘Tir’ (translocated intimin receptor) [5]. The interaction of these three factors is crucial in the pathogenicity of EHEC.
Once the bacteria enter the gastrointestinal tract, they penetrate the thick mucus layer that protects the enterocytes. Through T3SS, Tir infects the cells and is expressed on the surface of the enterocytes. The interaction between Tir and the adhesin intimin allows the bacteria to attach to the mucosal surface [5]. EHEC has now colonised the intestinal tract.
Strategy two: The attack
The colonisation of the bowel is only one part of the two-pronged strategy pursued by E. coli O157. This strain of E. coli is also known to produce a cytotoxin called Shiga toxin or Stx [1].
On the one hand, Stx increases the expression of nucleolin, which is another surface receptor for intimin, reinforcing the adherence of the bacteria to the mucosal surface.
On the other hand, once the bacterial cell is lysed, Stx is released into the intestinal lumen and binds to a Gb3 receptor on microvascular endothelial cells of the small intestine. The Stx-Gb3 complex is transported into the cell and towards the endoplasmatic reticulum.
Once it arrives, the complex inhibits protein synthesis, which leads to cell death [6, 7]. In case of sublethal concentrations, Stx can lead to a remodelling of cell expression. This can result in activation of platelets [8], expression of adhesion molecules [9] and inflammatory chemokines [10]. The cytotoxic effect of Stx is potentiated, promoting the adhesion of WBC to endothelial cells, causing thrombosis and tissue damage. Thrombosis in the capillaries subsequently leads to mechanical haemolysis [11], so patients present with fragmented RBC as well as decreased platelet counts.
References
[1] Tarr PI et al. (1995): Escherichia coli 0157:H7: Clinical, Diagnostic, and Epidemiological Aspects of Human Infection. Clin Infect Dis. 20(1): 1-10.
[2] Riley LW et al. (1983): Hemorrhagic colitis associated with a rare Escherichia coli serotype. N Engl J Med. 308(12): 681-685.
[3] Majowicz SE et al. (2014): Global incidence of human Shiga toxin-producing Escherichia coli infections and deaths: A systematic review and knowledge synthesis. Foodborne Pathog. Dis. 11(6): 447–455
[4] Gambushe SM et al. (2022): Review of Escherichia coli O157:H7 Prevalence, Pathogenicity, Heavy Metal and Antimicrobial Resistance, African Perspective. Infect Drug Resist. 15: 4645–4673.
[5] Joseph A et al. (2020): Shiga Toxin-Associated Hemolytic Uremic Syndrome: A Narrative Review. Toxins. 12(2): 67.
[6] Johannes L et al. (2010): Shiga toxins—From cell biology to biomedical applications. Nat. Rev. Microbiol. 8(2): 105–116.
[7] Kavaliauskiene S et al. (2017): Protection against Shiga Toxins. Toxins. 9(2): 44.
[8] Karpman D et al. (2001): Platelet activation by Shiga toxin and circulatory factors as a pathogenetic mechanism in the hemolytic uremic syndrome. Blood. 97(10): 3100–3108.
[9] Morigi M et al. (1995): Verotoxin-1 promotes leukocyte adhesion to cultured endothelial cells under physiologic flow conditions. Blood. 86(12): 4553–4558.
[10] Zoja C et al. (2002): Shiga toxin-2 triggers endothelial leukocyte adhesion and transmigration via NF-kappaB dependent up-regulation of IL-8 and MCP-1. Kidney Int. 62(3): 846–856.
[11] Bommer M et al. (2018): The Differential Diagnosis and Treatment of Thrombotic Microangiopathies. Dtsch Arztebl Int. 115(19): 327–334.


