Colorectal cancer awareness – prevention is the best treatment
Colorectal cancer (CRC) – also sometimes referred to as colon cancer, bowel cancer or rectal cancer – is the third most common cancer in the world and one of the leading causes of cancer-related deaths. In fact, it is the second most common cause of cancer death in both men and women in Europe [1]. One of the biggest things that sets colorectal cancer apart from other cancers is the ease and convenience of early screening and with this, the chance to reduce mortality across the globe.
Most colorectal cancers start as a polyp on the inner lining of the colon or rectum. Not all polyps become cancer, but some types of polyps can develop into cancer over time [2]. If detected at an early stage, a cancerous polyp can usually be removed, and the chance of advanced-stage cancer greatly reduced.
Early detection, individualisation of cancer therapy, and the use of less invasive diagnostics are leading to improved prognoses as well as patients’ quality of life [3]. Identifying the cancer early on is especially important for therapeutic success. Screening – both symptomatic and especially large-scale preventative screening – is essential. Utilising the FIT (faecal immunological testing) screening helps to find blood in stool, thus identifying even pre-cancerous lesions before they turn into cancer.
We are playing a key role in these improvements and everything we offer is uniquely designed to address specific needs of lab managers and clinicians, especially those who address the prevention, diagnosis and treatment of colorectal cancer disease. At the end of the day, we all have the same goal – improving the lives of cancer patients.
Colorectal cancer prevention with the FIT screening test
Early detection of colorectal cancer is key
Colorectal cancer (CRC) is increasing globally, but the potential for a full recovery is known to increase when it is discovered early. This is why early screening is extremely important.
One of the most reliable early-detection methods for colorectal cancer and its preliminary stages is the faecal immunological test (FIT).
A potential sign of colorectal cancer can be intestinal bleeding, which the FIT can identify – even in the smallest amounts of blood in the stool. If the findings are positive, further diagnostic actions can be undertaken at an early stage.
Various studies have shown that the detection rate for the FIT compares very well with colonoscopy. Our test and associated screening programme are already being used in several European national and regional colorectal cancer prevention programmes.
The FIT test kit (SENTiFIT pierceTube), stool sampling is easy, stress-free and can be performed comfortably at home. The sample is then analysed in a laboratory and the patient will be informed about his test results soon afterwards. Screening with the FIT test is simple and quick and no big deal except for the huge relief your patients feel afterwards.
We offer faecal immunochemical test (FIT) kits for two main audiences: large-scale screening programmes (rule-in testing) and testing of symptomatic patients (rule-out testing) in hospital or clinical routine settings.
Cancer diagnosis with OSNA
Cancer staging is the step that determines the type and extent of treatment. An essential part of this staging process is assessment of the nodal status.
Revealing the whole picture
Lymph node status is the most important prognostic factor and staging parameter in colorectal cancer management. The current histopathological method consists of the analysis of less than 2% of the total lymph node tissue. As a result, metastases may remain undetected, leading to false-negative results and a higher risk of disease recurrence.
OSNA (one step nucleic acid amplification) allows fast, sensitive and standardised lymph node analysis, enabling accurate staging and avoiding false-negative results. By analysing the entire lymph node, the OSNA method provides a reliable basis for decisions on adjuvant therapy, avoiding understaging and thus preventing undertreatment of patients.
OSNA improves patients’ quality of life by providing an accurate and fast diagnosis, reducing risk of undertreatment and decreasing overall patient stress.
For more information on our OSNA solution visit our website.
Liquid biopsy for monitoring
Analysing genetic mutations in biopsied tissue has been the standard in clinical oncology, despite its inconvenience and risks of clinical complications for the patients.8 Moreover, biopsies are subject to selection bias due to tumour heterogeneity, can be difficult to obtain in sufficient amounts and the information provided is restricted to a static time point.9,10 Here, liquid biopsy analysis of mutations in cell-free tumour DNA (ctDNA) has become a valuable tool for a variety of clinical and investigative applications. The detection of clinically relevant mutations from a simple blood draw enables clinicians to select the most beneficial therapy and monitor the patient’s response to therapy in real time.
* SureSeqTM myPanelTM: For Research Use Only. Not for use in diagnostic procedures.
Manufacturer and Trademarks: CytoCell® FISH Probes (Cytocell Limited), SureSeqTM myPanelTM (Oxford Gene Technology IP Limited)
CRC treatment solutions - precisely brings to light what needs to be seen
Precision diagnostics are key to selecting the right therapy at the right time for the right cancer patient.
Gene alterations play a key role during the onset and development of colorectal cancer.6 Therefore, reliable assessment of the tumour’s mutational status is decisive not only in selecting cancer patients eligible for targeted therapy, but also later on, to monitor response to treatment and disease progression.
Nowadays, a range of medications targeting tumour-specific genetic alterations are available, and can be prescribed instead of, or in combination with, standard chemotherapies and radiotherapies. To investigate such genetic changes, oncologists can make use of a variety of molecular genetic tests which may support them in making personalised treatment decisions.
Sysmex offers a range of products based on cutting-edge molecular biology technologies supporting heath care professionals and patients along the whole pathway of care.
Molecular tests for therapy selection
With CytoCell FISH (Fluorescent In Situ Hybridization) probes for MET and EGFR genes, oncologists will get specific information on colorectal cancer prognosis and indication for EGFR-inhibitor medications.7
Other critical and currently relevant colorectal cancer genes including KRAS, NRAS, APC, TP53, ERBB2, PTEN and BRAF can be investigated by Next Generation Sequencing tests, with the Sysmex solution in this case being the SureSeq myPanelTM* NGS Custom Colorectal Cancer Panel.
For further information visit SureSeq myPanel™ NGS Custom Colorectal Cancer Panel website
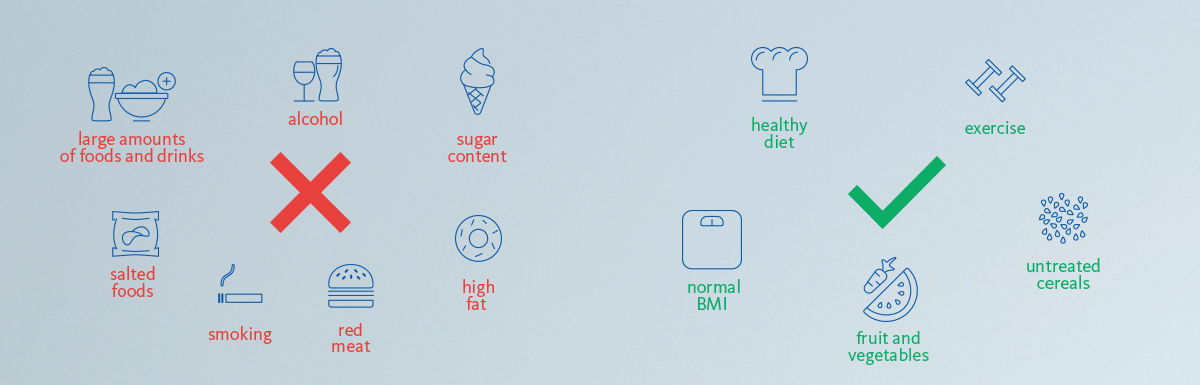
What can we do to prevent risk of developing colorectal cancer?
Despite the fact that colorectal cancer risk is increased by older age, there has been an uptick of diagnoses of younger patients in recent years [4]. Other risk factors include incidence of colorectal polyps, genetics, unhealthy lifestyle and chronic inflammatory bowel diseases.
An unhealthy lifestyle can also increase the risk of not only cancer, but many other diseases as well.
Here are some healthier habits we can all practice to make sure our colorectal health is in order [5]:
- Consuming a healthy diet
- Avoid consuming large amounts of foods and drinks with high fat and sugar content. Opting for foods and drinks that are low in sugar but high in fibre is the healthier option.
- Avoid consuming large amounts of red meat, including pork, beef, lamb and goat meat.
- Avoiding heavily salted foods and drinks. The recommendation is to not exceed 5–6 grams of salt per day.
- Eating five portions of fruit or (non-starchy) vegetables a day are recommended, as well as adding untreated cereals or pulses to every meal.
- Having a healthy body weight and exercising regularly
- Drinking as little alcohol as possible
- Not smoking, as tobacco is one of the main causes of cancer
- Perhaps most importantly, taking part in early detection programmes
- Some kinds of cancer can be detected and treated before any symptoms appear.

References
- World Health Organization. https://www.euro.who.int/en/health-topics/noncommunicable-diseases/cancer/news/news/2012/2/early-detection-of-common-cancers/colorectal-cancer
- American Cancer Society. https://www.cancer.org/cancer/colon-rectal-cancer/about/what-is-colorectal-cancer.html
- Lee J.J. et al. (2016): Options for Second-Line Treatment in Metastatic Colorectal Cancer. Clinical Advances in Hematology & Oncology, 14(1):46-54.
- Yale Medicine. (2022): https://www.yalemedicine.org/news/colorectal-cancer-in-young-people
- International Agency for Research on Cancer. https://www.iarc.who.int/
- Ogunwobi, O.O.; Mahmood, F.; Akingboye, A. Biomarkers in Colorectal Cancer: Current Research and Future Prospects Int. J. Mol. Sci. 2020, 21, 5311
- Hegymegi-Barakonyi B, Tyrosine kinase inhibitors - small molecular weight compounds inhibiting EGFR Curr Opin Mol Ther 2009;11(3):308-21
- Overman MJ, Modak J, Kopetz S, et al: Use of research biopsies in clinical trials: Are risks and benefits adequately discussed? J Clin Oncol 31:17-22, 2013
- Vogelstein B, Papadopoulos N, Velculescu VE, et al: Cancer genome landscapes. Science 339:1546-1558, 2013
- Gerlinger M, Rowan AJ, Horswell S, et al: Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med 366:883-892, 2012